Investment to expand cell line development capabilities and accelerate customer workflows, as part of its diverse and tailored antibody solutions.

Cambridge, UK, and Porto, Portugal, 03 October 2023: Sphere Fluidics, a company developing single cell analysis systems underpinned by its patented picodroplet technology, today announced the Cyto-Mine® platform has been selected by FairJourney Biologics, a world leading end-to-end antibody partner, providing integrated services across antibody discovery, engineering, and production, to expand its cell line development capabilities. FairJourney Biologics has adopted the platform as part of the launch for its latest cell line development services, to streamline and accelerate customer workflows.
FairJourney Biologics’ team of experts comprises an extensive knowledge of cellular biology, enabling them to tailor cell lines precisely to their partners’ unique requirements. Leveraging state-of-the-art technologies and meticulous quality control measures, FairJourney Biologics consistently delivers high-performing, stable cell lines that serve as the foundation for efficient antibody production and therapeutics development. Cyto-Mine® ‘s advanced features, such as selection of producer clones, monoclonality assurance, significant time savings, flexible assay designs, GLP compliance support, and regulatory compatibility, seamlessly complement FairJourney Biologics’ services, providing partners with comprehensive and efficient solutions for their research and development endeavors.
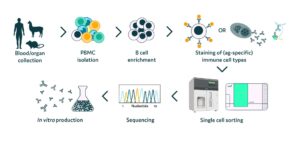
Traditional processes for finding novel antibody targets or developing stable cell lines are typically time-consuming, often inefficient, and difficult to scale. Cyto-Mine® is an automated platform which integrates single cell screening, sorting, dispensing, imaging, and clone verification, with proof of monoclonality. Underpinned by Sphere Fluidics’ patented, microfluidic picodroplet technology, it provides an integrated system with an easy-to-use and intuitive interface that can automatically, yet gently, screen up to 40 million cells in a matter of hours, compared with 10,000 typically achieved using multi-step manual techniques. This accelerated throughput is already widely recognized across a variety of research areas, including antibody discovery, cell line development, cell engineering and synthetic biology. The platform also facilitates rapid, high-throughput single cell manipulation and analysis across an expanding range of emerging research areas, including precision genome editing, cell therapy research and cellular diagnostics.
FairJourney Biologics’ partners will also benefit from the Cyto-Mine® ’s recent software updates, supporting FDA 21 CFR Part 11 compliance and including a full Installation Qualification / Operation Qualification (IQ/OQ) package for pharmaceutical quality assurance, extending the system’s applications into drug manufacturing operations1.
Richard Hammond, Chief Technical Officer, Sphere Fluidics, said: “The FairJourney Biologics team has an extensive track record of expertise across antibody discovery, engineering, and production, enabling them to solve unique and challenging projects. We feel proud that the Company has recognized the potential of the Cyto-Mine® to advance its cell line development workflows and the benefits it can give to customers. We have worked closely to support the system’s integration and will continue to provide technical support as needed; we very much look forward to successes to come from FairJourney Biologics’ new services.”
António Barroso, Head of Cell Development Division, FairJourney Biologics, commented: “We are committed to providing the tools and resources needed to advance scientific research and accelerate drug discovery and development. Our new service has been purpose-built to help researchers and scientists in these fields achieve their goals with greater efficiency, accuracy, and reliability. The integration of Sphere Fluidics’ Cyto-Mine® platform into our cell line development workflows enables us to best cater to our partners’ needs, to provide high-quality, customized cell lines in a fraction of the time.”
- Press release (5th July, 2023) Sphere Fluidics expands Cyto-Mine® capabilities to meet cGMP requirements for drug manufacture workflows
For further information please contact:
FairJourney Biologics
Ana Meneses, Head of Business Development
Email: info@fjbio.com
Sphere Fluidics
Dr. Claire Cox
Tel: +44 (0)7365 209 441
Email: claire.cox@spherefluidics.com
Zyme Communications
Anna Bakewell
Tel: +44 (0)7891 477 378
Email: anna.bakewell@zymecommunications.com
To opt-out from receiving press releases from Zyme Communications please e-mail info@zymecommunications.com. To view our privacy policy, please click here.
About FairJourney Biologics
FairJourney Biologics is a world leading end-to-end antibody partner, providing integrated services across antibody discovery, engineering, production and characterization to global biopharma. With extensive expertise and know how in antibody discovery and development, FairJourney Biologics has become the go-to-partner for successful antibody campaigns, with at least 14 antibodies currently advancing to clinical stages as a direct outcome of partnerships. The Company’s significant expertise in phage display technology, combined with a diverse approach to generating both immune and naïve antibody libraries have contributed to a market leading 99%+ project success rate.
For more information, please visit: www.fjbio.com
About Sphere Fluidics
Sphere Fluidics’ platforms are built on over a decade of scientific and engineering research and development. Originally spun out from the University of Cambridge, we specialize in multiple technical areas to deliver miniaturization.
For further information contact: